Notice: Morris Bart is no longer accepting new cases related to Philips DreamStation CPAP.
Philips has manufactured, marketed, and sold the DreamStation and similar CPAP, BiPap, and ventilator units using a defective sound abatement foam since 2009. Recent studies have shown that foam degradation has led to various negative health side effects in users of Philips devices. However, it has now been over two years since the recall of the defective products, which means, in most cases, the statute of limitations to file claims has passed.
What Is a Philips CPAP Machine?
CPAP is an acronym that stands for Continuous Positive Airway Pressure. First designed in 1980, CPAP devices are primarily designed to assist sleep apnea sufferers. By sending a steady flow of oxygen to the mouth and nose of a sleeping user, the device can keep airways open, thereby keeping the user breathing normally. These are slightly different than Bi-Pap machines, which have two pressure settings, and ventilators, which are a wider range of assisted breathing devices.
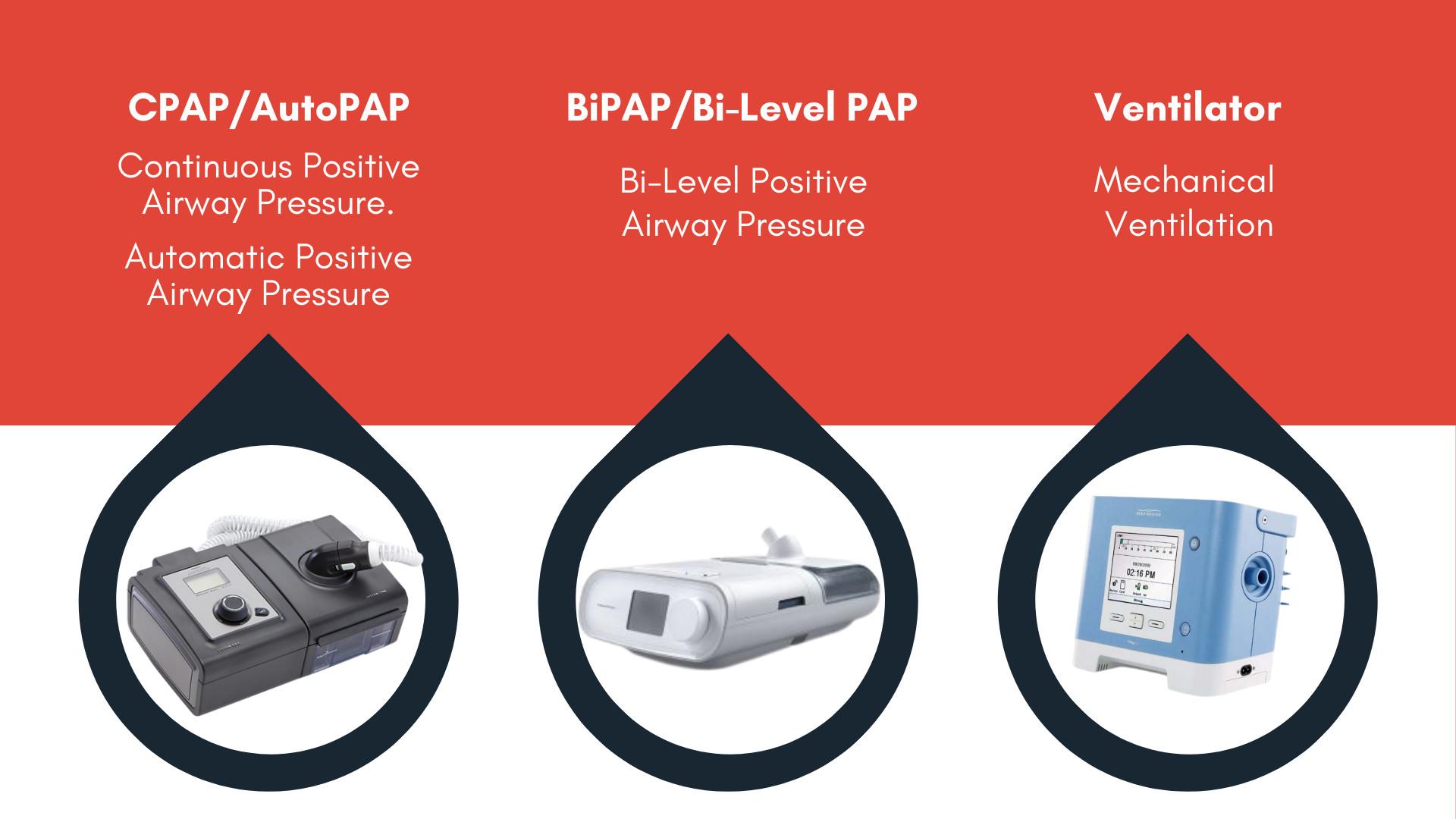
Has There Been a Recall on Philips CPAP Devices?
Yes. In June 2021, Philips recalled certain Respironics ventilators, BiPAP, and CPAP machines. The FDA has also issued a safety communication with more information regarding the specific devices recalled.
While more recalls may occur, below is a list of recalled CPAP, BiPAP, and ventilator machines.

What Issues Does the Philip CPAP Cause?
While the individual cases can vary, patients with Philips CPAP devices have a wide variety of potential risks due to foam degradation. Beyond potential inhalation or swallowing of PE-PUR foam present in the devices, foam degradation can also lead to “outgassing.” This means the foam breakdown can result in the release of toxic gasses, which can cause many other issues if inhaled. Examples include:
- Various cancers
- Respiratory distress or failure
- Heart attack
- Chemical burns
- Brain damage
- Cellular damage to DNA
What Kind of Cancers Is Breathing Polyurethane Foam Associated With?
Unfortunately, there are a variety of cancers that are tied to the breathing of vapors released through polyurethane foam degradation, including:
- Stomach Cancer
- Testicular Cancer
- Thyroid Cancer
- Acute Respiratory Distress System (ARDS)
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Hematopoietic Cancer
- Kidney Cancer
- Leukemia
- Liver Cancer
- Liver Damage
- Lung Cancer
- Rectal Cancer
- Lymphatic Cancer
- Multiple Myeloma
- Nasal Cancer
- Non-Hodgkin Lymphoma
- Papillary Carcinoma
- Prostate Cancer
How Many Philips CPAP Devices Have Been Recalled?
It is estimated that 2 million to 3 million different Philips CPAP, Bi-PAP, and other ventilation devices have been recalled. Nearly two-thirds of these devices have been sold within the United States.
Am I Eligible for Compensation?
Patients who have been using a CPAP or BiPAP device from Philips may be eligible for compensation. However, Philips has announced a recall and taken steps to notify users of its products’ defects.
Most states carry a 2-year statute of limitation for all product liability claims. While certain exceptions certainly apply, this means that most CPAP users who have not already filed a claim may be unable to be compensated.